Turning Sand into fuel - Silicon oil as an energy carrier
Dr Peter Plichta studied chemistry, physics and nuclear chemistry in Cologne, Germany. He obtained his doctorate in chemistry in 1970, and in the years following he did much research, on the subject of silanes. Similar to hydrocarbons, silanes are hydrosilicons, molecules that incorporate atoms of both silicon and hydrogen.

Plichta also studied law, and in the 1980s he studied and researched logics, numbers theory and mathematics. As a result, he published several books outlining a new theory on prime numbers in German. In this article however, I will only discuss his proposal to use silanes as a highly energetic fuel.
Silicon is more abundant than carbon. It oxidizes or combines with oxygen into silicon dioxide, which forms crystals present in rocks like quartz, basalt and granite. Silicon dioxide is especially prevalent in sand which fills deserts and sea shores. We process silicon dioxide into glass and purify the silicon for use in electronics. Both of those processes require much external energy input.
Before the 1970s, silanes were considered unsuitable for use as fuels, because they instantaneously self-combust at room temperature. Not satisfied to leave it at that however, Plichta went to work and succeeded in producing longer-chained silanes that appeared as clear, oily liquids and were stable at room temperature. He argues that these higher (long-chain) silanes could be used as an abundant fuel as an alternative to both hydrocarbons and pure hydrogen.
Unlike hydrocarbons, silanes use both the nitrogen and the oxygen in air for combustion. While the hydrogen component of silanes reacts with oxygen, the silicon oxidizes in a highly energetic reaction with nitrogen. So the burning of silanes produces much higher temperatures and frees more energy than the burning of hydrocarbon fuels. The silane reaction leaves no toxic residues.
Much of the information in this article comes from a recent description of Plichta's discoveries and his proposed silane fuel cycle written by Norbert Knobloch and published in the German magazine raum&zeit.

If you read German, you can see the original article in pdf format here.
Dr. Plichta's website, also in German, has much additional information.
- - -
Peter Plichta's book "Benzin aus Sand" (Gasoline from Sand), first published in 2001, advocates a change in energy strategy away from burning hydrocarbons to using the energy potential of silanes or, as I would term them, hydrosilicates.

The book, so far only in German, is available from Amazon.
But let's get down to the nitty gritty details, to get a better idea what is being proposed and is being discussed, confidentially for now, with international investors.
Nitrogen oxidizes silicon
Silicon is the most abundant element in the earth's crust. Combined with hydrogen, silicon forms what in chemistry are known as "silanes". Given sufficient heat, silanes react with the nitrogen in the air. This is a new discovery. Nitrogen was thought to be inert, as far as combustion is concerned. So we obviously must re-think the possibilities of combustion. Silicon makes up 25% of the earth's crust, while nitrogen makes up 80% of air. A process that uses silicon/nitrogen combustion in addition to the known carbon/oxygen cycle, presages some mind boggling new possibilities.
While carbon is also a relatively abundant element, its prevalence is way lower than that of silicon. The relation is about a hundred to one. In addition, most of the available carbon is bound up in carbonaceous minerals such as marble and other carbon-based rocks and some of it is in the atmosphere as carbon dioxide. Those forms are not available for use in the combustion cycle. Only one in about a hundred thousand carbon molecules is bound to hydrogen, making it available for the purpose of combustion. So while carbon has served us well for the first century and a half of industrialization, it is a rather limited fuel.
Using 100% of air for combustion
Plichta's idea was to exchange chains of carbon atoms in hydrocarbons for chains of silicon in hydrosilicons or silanes. The long chained "higher silanes" are those with five or more silicon atoms in each molecule. They are of oily consistency and they give off their energy in a very fast, highly energetic combustion.
While hydrocarbon-based gasoline only uses oxygen, which makes up 20% of air, for their combustion, the hydrosilicon-based silanes also use nitrogen, which makes up the other 80% of air, when they burn. Silanes with chains of seven or more atoms of silicon per molecule are stable and can be pumped and stored very much like gasoline and other carbon-based liquid fuels.
The efficiency of combustion depends on the amount of heat that is created. Expanding gases drive pistons or turbines. When hydrocarbons are burned with air as the oxidant, efficiency of combustion is limited by the fact that the 20% of air that partakes in the combustion also has to heat up the nitrogen gas, which isn't participating but has to be expanded as well. When burning silanes, practically all of the air participates directly in the combustion cycle, making for a much more efficient expansion of all the gases involved.
Burning silanes
The combustion process of hydrosilicons is fundamentally different from the exclusively oxygen based combustion we know from burning hydrocarbons. In a sufficiently hot reaction chamber, silanes separate into atoms of hydrogen and silicon, which immediately mix with the oxygen and nitrogen of the air. The hydrogen from the silanes and the air's oxygen now burn completely leaving only water vapor, bringing the temperature of the gases close to 2000 degrees C.
Since there is no more oxygen, no silicon oxide can be formed in the following phase. What happens instead is an extremely energetic reaction of the 80% nitrogen in the air with the silicon atoms present, that forms a fine powder called silicon nitride (Si3N4).
For those more technically inclined, taking the example of hexasilane (Si6H14), here is what the reaction would look like:
• 2 Si6H14 + 7 O2 + 8 N2 -> 4 Si3N4 + 14 H2O
After this first reaction, a great deal of unreacted nitrogen is still in the combustion gases, which would now react in a stochiometric combustion as follows:
• 4 1/2 Si6H14 + 18 N2 -> 9 Si3N4 + 63 H
Overall, on the input side of the equation we would have:
• 6 1/2 Si6 H14 + 7 O2 + 26 N2
and on the output side, we get:
• 14 H2O + 13 Si3N4 + 63 H
The silicon nitride we find in the "exhaust" is the only known noble gas that exists in solid form, an original discovery by Peter Plichta. That white powdery stuff is a rather valuable raw material for ceramics.
Wikipedia says that silicon nitride powder will form
"... a hard ceramic having high strength over a broad temperature range, moderate thermal conductivity, low coefficient of thermal expansion, moderately high elastic modulus, and unusually high fracture toughness for a ceramic. This combination of properties leads to excellent thermal shock resistance, ability to withstand high structural loads to high temperature, and superior wear resistance. Silicon nitride is mostly used in high-endurance and high-temperature applications, such as gas turbines, car engine parts, bearings and metal working and cutting tools. Silicon nitride bearings are used in the main engines of the NASA's Space shuttles."
Rocket fuel for space propulsion
One of the first uses Peter Plichta envisioned for these long-chain hydrosilicons he discovered was to be a fuel for rockets. Space travel today is hindered by the immense weight of fuel a rocket has to carry to lift itself plus the fuel, plus its payload, into space. With a more efficient combustion process, and an oxidant that could be "scooped up" in the atmosphere, a disk-shaped craft could be propelled to great speed and altitude, before having to fall back on a rather small amount of oxidant that may be carried as liquefied air or liquid nitrogen.
I found a discussion of this on the net, here, which I reproduce below in shortened and slightly edited form:
http://discaircraft.greyfalcon.us/Richard%20Miethe.htm"Dr Plichta can use his concepts of cyclic mathematics to effect a revolution in space travel. He has already received several patents for the construction of a disc-shaped reusable spacecraft which will be fueled by the diesel oils of silicon. The special feature of these carbon analog substances is that they do not only burn with oxygen, but also with nitrogen. Such a spacecraft can use the atmosphere for buoyance. Its engines can inhale air and thus do without the standard oxidant reservoir.
In 1970 Peter Plichta disproved the textbook theory that the higher silanes are unstable. One of his achievements was to create a mixture of silanes with the chain lengths 5 to 10 (Si5H12 to Si10H22). He also managed to separate the oil into the individual silanes by of means gas chromatic analysis. This showed the surprising result that silanes with a chain length of over 7 silicon atoms will no longer ignite spontaneously and can thus be used for commercial purposes.
Multi-stage rockets function from the mathematical point of view according to principles of rocket ascent. At the first stage of the launch they have to lift their whole weight with the power of fuel combustion. Because they quickly lose weight as they use up fuel, they then accelerate although the power of thrust remains the same. The discarded stages are burned in the atmosphere, which can only be described as a ridiculous waste of money. The Space Shuttle was intended to make space travel less costly; but actually the opposite has happened. Just as the invention of the wheel made all human transport easier, a circular spacecraft will some day soon replace the linear design of current multi-stage rockets. We are all familiar with the elegance with which a disc or a Frisbee is borne by the air through which it flies.
Peter Plichta got the idea of constructing a disc in which jet-turbines attached to shafts would drive two ring-shaped blade rings rotating in opposite directions. This will cause the disc to be suspended by the air just like a helicopter. The craft can then be driven sideways by means of a drop-down rocket engine. When a speed of over 200 km/h has been reached, the turbines for the blade rings will be switched off and covered to enhance the aerodynamic features of the shape. The craft will now be borne by the up-draught of the air, just like an aircraft is. This will also mean that the critical power required for rocket ascent will not be necessary. When the spacecraft accelerates into orbit, the N2/O2 mixture of the air will first be fed in through a drop-down air intake, as long as the craft is still at a low altitude of 30 km (1 per cent air pressure). The air will be conducted to the rocket motor and the craft will thus accelerate to a speed of 5000-8000 km/h. This is where a standard rocket jettisons its first stage, because by then about 75% of the fuel has already been used up.
The disc on the other hand will continue to accelerate to 20,000 km/h and will thus reach an altitude of about 50 km (1 per thousand of air pressure). The speed will increase as the air pressure drops, so that the process can be continued until an altitude of about 80 kilometers and 25,000 km/h can be maintained. In order to reach the required speed of 30,000 km/h and an altitude of around 300 km, only a relatively small quantity of oxidation agent will be needed at the end.
In the hot combustion chamber silanes decompose spontaneously into hydrogen and silicon radicals. The hydrogen is burned by the oxygen in the air and water formed. Because molecular nitrogen is very tightly bonded, it must be preheated and subjected to catalytic dissociation. The extremely hot silicon radicals will provide additional support for this process, which will in turn lead to silicon nitride being formed. In order to burn superfluous nitrogen, Mg, Al or Si powder can be added to the silane oil.
When the spacecraft returns from space the ceramic-protected underside of the disc will brake its speed to approximately 500 km/h. Then the covering will open again, making the blade rings autorotate. The jet turbines will then be started for the actual landing operation.
In 2006, Plichta developed a new low-cost procedure for the production of highly purified silicon. This makes it possibile to hypothesize a more widespread use of silanes. If widely and cheaply available one day, the new fuel could be used in turbines and modified internal combustion engines, in addition to space rocket use.
Large-scale production of silanes
In order to use long-chain silanes as a fuel, the possibility of large scale production of those silicon oils will have to be experimentally confirmed. According to Plichta, this process would also involve production of pure silicon for use in photovoltaic or other industrial applications. High grade energy is needed to transform silicon oxide into pure silicon, to be hydrated producing the silanes.
One possibile way to go about this is to use photovoltaic electricity to disassociate hydrogen and oxygen from water. Those gases could then be used to process sand into pure silicon and to obtain silanes.
Another procedure, widely used today, is to purify silicon dioxide using heat from coal, but Plichta has now developed a new process that would use tar, pitch and bitumen as well as aluminium silicate to produce pure silicon and silanes at a very low cost. The highly exothermic process produces large amounts of hydrogen and it involves super heated hydrogen fluoride. Monosilanes, a by-product of this new process, could be reacted with carbon dioxide to obtain water and silicon carbide, an extremely hard substance and industrial raw material.
Details are still confidential. The process is being patented.
Turbines and engines
Since the silane combustion process is substantially different from that of the hydrocarbons used today, specially designed turbines and engines will be needed to make use of the new fuel. Dr Plichta has patented a turbine that would optimally use the silicon-based combustion process.
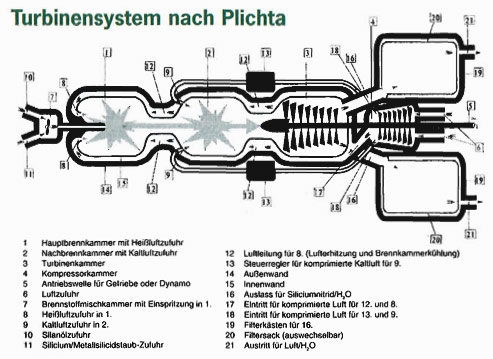
A mixture of silane oil (10) and silicon powder (11) are mixed and injected by a pump (7) into the main combustion chamber. There the fuel is burned together with pre-heated air (8). In the secondary combustion chamber (2) the fuel mix is further burned with a large amound to cold air (9), quickly lowering the temperature of the gases from about 2000 degrees C to a few hundred degrees. This brings a large pressure increase. If the silicon nitride powder produced by the combustion process were too hot and not diluted with air, it would destroy the turbine blades.
The resulting mixture of gases (H2O, O2, and Si3N4 of oily consistency) is now able, in the turbine chamber (3), to cause the turbine blades to rotate. The rotation is transmitted over a connected shaft (5) to the compressor chamber (4) where air is aspired through air inlets (6). The air is mostly conducted into the secondary combustion chamber (2) and a small part of it goes, after heating, to the first combustion chamber (1). The the absorption of heat by the air also provides needed cooling of the combustion chambers.
The water vapor produced by the combustion process leaves the turbine through exhaust openings (21) while the cooled down, solid silicon nitride is trapped in dust bags (20), ready to be passed on for later industrial uses.
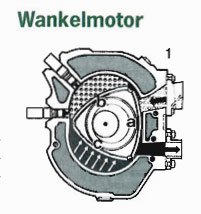
Internal combustion engines of the Otto and Diesel type would suffer breakdown of lubrication if made to burn silicon oils. The temperatures of combustion are considerably higher than those reached by gasoline or diesel. But according to Plichta, the Wankel-type rotary piston motor could be modified to accomodate the high temperatures. It parts would have to be coated with silicon nitride ceramics or be entirely constructed using the even harder silicon carbide.
The silane oils could not be compressed together with air, they would have to be injected at the point of maximal compression. The silicon nitride contained in the combusting fuel/air mixture would initially be in gaseous and liquid form, providing the necessary lubrification and acting as a sealant. Exhaust gases, still very hot, could be further burned in a turbine, with the addition of cold air as in the second stage of Plichta's turbine design.
Like in the turbine, combustion in this engine would produce small amounts of silicon nitride in powder form, which would be filtered out from the exhaust gases and collected by filling stations, to be passed on for industrial uses.
Solar energy and silanes - closing the circle
Solar energy can be transformed into electricity without much trouble, but not everything in this technological world can be run with electricity. Storage is a problem as battery technology definitely is not up to the task yet. One way around that is to produce hydrogen with solar energy and use the hydrogen as a fuel. This is problematic because of the volatility and the relatively low energy density of molecular hydrogen.
Bringing silicon into this cycle would allow us to continue using liquid fuels where needed, and given that silanes store energy at a higher density than hydrocarbons, and definitely at a higher density than pure hydrogen, this may be a good route to choose.
There are no byproducts of this cycle that would have to be vented into the environment and be destructive. The principal "exhaust gas" from silane combustion, silicon nitride, is a valuable industrial raw material that can easily be collected and recycled into technical and construction uses.
In case there would be "too much of a good thing" or an overabundance of silicon nitride, the powder could also be chemically transformed using sodium hydroxide (NaOH) or potassium hydroxide (KOH). The transformation would produce ammonia (NH3) and water soluble silicates. The silicates are non-toxic and will degrade in ambient air to form sand crystals.
Although ammonia is atoxic gas, since it burns without any toxic residues and without carbon emission, it could be used in the production of further energy, or even as a fuel in cars, as proposed by nh3car.com. Burning ammonia with air produces steam and pure nitrogen.
• 4 NH3 + 3 O2 -> 2 N2 + 6 H2O
Other uses for ammonia would be the production of nitrogen rich fertilizer, dynamite or household ammonia which is ammonia diluted in water.
The complete solar/silane cycle would involve the production of pure silicon from sand, either using solar energy or tars and bitumens. The next step is the synthesis of higher silanes. Plichta proposes to use a modified high pressure Muller-Rochow synthesis for this step. Then silanes could be burned in modified turbines and engines, or used in space propulsion systems. The fourth step is the re-cycling and re-use of the principal product of combustion, silicon nitride. What is not used industrially, can be chemically transformed into ammonia, which again produces nitrogen which was used in step 3 for combustion.
The pure silicon produced in step 1 would be of use in the production of more and cheaper solar panels to more efficiently capture the sun's free energy.
Dr Peter Plichta may be contacted through his website at
http://www.plichta.de/pp24/index.php?option=com_contact&Itemid=7
Comments
March 16, 2010 5:36 PM | Posted by: Ernst von Bezold
If hydrosilicon combustion may become an economic fact, what would or could be the enviromental health and other environmental impact risks, of, e.g., pulmonary silicosis, carcinogenicity, reproductive, immunologic, of respirable silicon nitride dust from the fuel combustion process; and also of consumption and seeding of the high planetary atmosphere by silicon nitrides?
Unless filtered perfectly, we should anticipate traces of it in the remaining air all over the planet.
Beyond direct weather and climate effects of atmospheric seeding and adverse health impacts associated with respirable silicate exposures, I wonder from a comparative example whether there may be other effects of highly dilute finely dispersed materials which we can consider early in this development. Rudolf Steiner suggested early in the 20th c. the importance for [terrestrial carbon-based] life of atmospheric traces of formic acid produced by ants.
In this respect how might large scale implementation of hydrosilicon combustion change the planetary biochemistry?
December 28, 2015 12:52 AM | Posted by: The Pilsner Prophet
Ernst: Didn't the article state that the dust is filtered out, caught, and saved? And what climate effects? There is NO climate effect from people of any kind! Go ahead and prove me wrong. Hint: insults and implications about my inteligance are not proof. Oh, by the way-our heavy-handed EPA will ensure there will be no change in planetary bio-chemistry. Unless, of course barack's buddies stand to make money. Remember solyndra? I know, I know, that wasn't about the environment. But it was about corruption of green energy policy.
January 29, 2016 6:23 AM | Posted by: Chris Skinner
As if all those political extremist bigots and hypocrites of the far right-wing(hellbent on maintaining the status-quo)never did persecute or suppress new inventions and technology with closed-minded arrogance and ignorance themselves.
However,The Pilsner Prophet is correct about how the concerns by Ernst are no big deal.Too many naysayers these days.
However,use of solar energy and photovoltaics is way too slow.You could initially in the short-term "ramp-up" the silane production infrastructure much faster,using an improved type of nuclear,such as the LFTR(Liquid Flouride Thorium Reactor).It could mass produce hydrogen much faster.After that,you could then use the same LFTR and silane infrastructure in the long-term to mass produce all the silicon solar cells you want.
June 7, 2016 10:52 PM | Posted by: Ed Wood
I am all for new technologies. I however have a couple of problems with silanes. mostly safety they can be quite explosive. Next I share Ernst von Bezold's concerns about large scale use. I was looking into Silanes a few years back in pursuit of a battery/fuel Cell. I got into the health hazards of silanes and diborane.
My only other concern is whether it would be cost effective. Silicon can be made easily I liked my cheap and dirty method burning Sand and magnesium dirty but would probably be cleaner with pure SIO2 and Magnesium powders. cheap and quick. I don't know if that would be really cost effective though. anyway I am interested in this and will look into it further.
June 10, 2016 7:10 AM | Posted by: Gary Eickmeier
Pardon me, but I think it safe to say that an idea that proposes to use sand as a fuel ingredient is as nutty as it gets. There have been documentaries on the scarcity of sand and its overuse in making concrete structures everywhere. If you think oil is a limited resource, take a look at sand.
July 14, 2016 3:42 AM | Posted by: Harmon Gladding
Silane fuel appears to show great promise but, unless the dust management cycle is absolutely perfect in design and operation, this extremely fine silicon nitride powder will be released.